उत्पाद विवरण
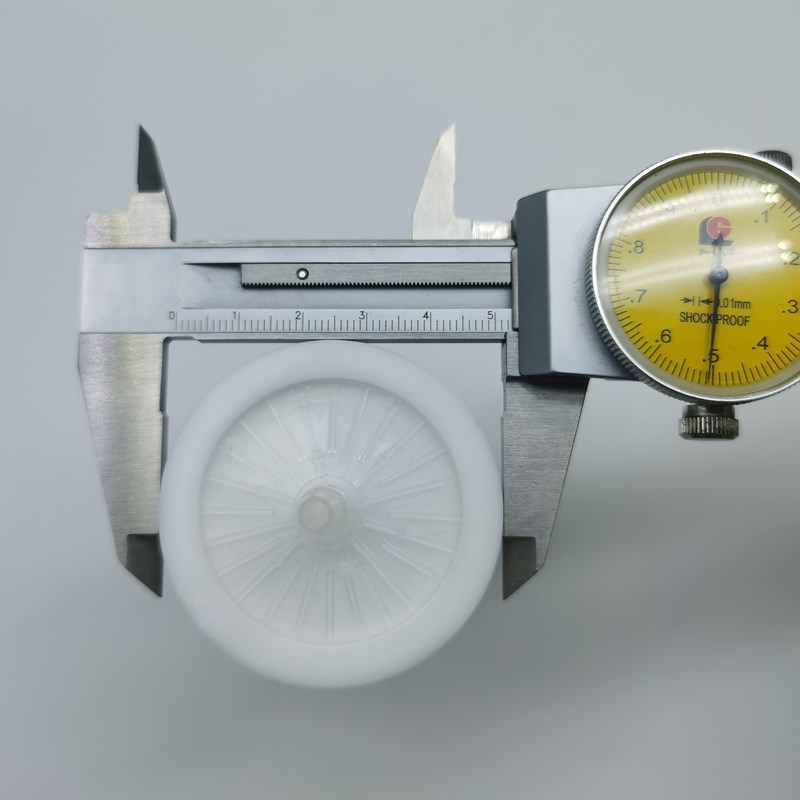
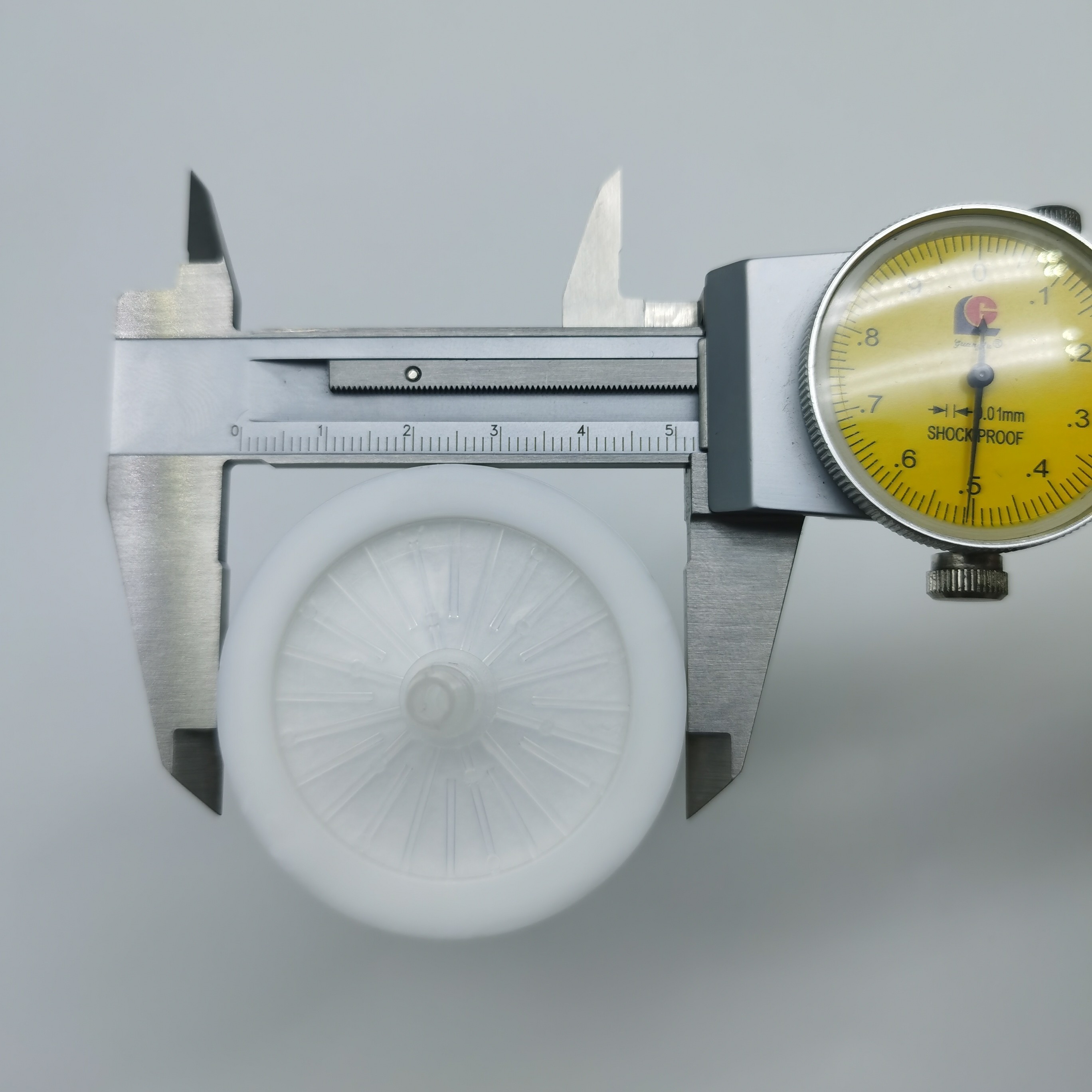
सक्शन यूनिट एस्पिरेटर के लिए 40 मिमी 0.2 माइक्रोन पीटीएफई हाइड्रोफोबिक बैक्ट्रिया फ़िल्टर
बैक्टीरिया फिल्टर का निर्माण पॉलीटेट्राफ्लुओरोएथिलीन (पीटीएफई) झिल्ली से किया गया है, जो कार्बनिक और अकार्बनिक रासायनिक संक्षारण, साथ ही अंतर्निहित हाइड्रोफोबिसिटी दोनों के लिए उत्कृष्ट प्रतिरोध प्रदान करता है। इसका व्यापक रूप से जैव प्रौद्योगिकी, फार्मास्युटिकल उद्योगों, प्रयोगशालाओं और स्टेराइल वेंटिंग की आवश्यकता वाली किसी भी प्रक्रिया में उपयोग किया जाता है। उत्पाद हल्का, सुरक्षित और विश्वसनीय है, इसमें ट्यूब के सिकुड़ने का कोई जोखिम नहीं है जो वेंटिलेशन में बाधा उत्पन्न कर सकता है।
उत्पाद की विशेषताएँ:
- स्वाभाविक रूप से मजबूत हाइड्रोफोबिसिटी, ऑक्सीकरण प्रतिरोध, और कार्बनिक/अकार्बनिक रासायनिक संक्षारण प्रतिरोध
- उच्च प्रवाह दर और कम निष्कर्षण
- आसान स्थापना और हटाने के लिए हल्के डिजाइन
- 100% अखंडता का परीक्षण किया गया
अनुप्रयोग:
- संस्कृति वाहिकाओं और सीओ का बाँझ निकास₂इनक्यूबेटर
- तरल कल्चर मीडिया का स्टेराइल वेंटिंग
- छोटे पैमाने के किण्वकों का बाँझ निकास
- भंडारण टैंकों का स्टेराइल वेंटिंग
- आटोक्लेव वेंटिंग
- गैस कण हटाना

विशेष विवरण
फ़िल्टर मीडिया: हाइड्रोफोबिक पीटीएफई को पॉलीप्रोपाइलीन के साथ लेमिनेट किया गया
निस्पंदन: 0.22μएम
आवास: पॉलीप्रोपाइलीन
आवास व्यास: 52 मिमी
कनेक्टर्स: 6 मिमी से 12 मिमी तक कठोर कदम
आवास की ऊँचाई: 54 मिमी
फ़िल्टर मीडिया व्यास: 40 मिमी
बंध्याकरण: 121 पर आटोक्लेव℃30 मिनट तक 60 चक्र
इस उत्पाद का विकास, उत्पादन और बिक्री प्रक्रियाएं ISO 9001 गुणवत्ता प्रबंधन प्रणाली आवश्यकताओं का अनुपालन करती हैं।
गुणवत्ता आश्वासन दिशानिर्देश
निम्नलिखित नियमित निरीक्षण आइटम हैं:
जैव
इस उत्पाद की सामग्रियों का परीक्षण किया गया है और VI-121 ℃ प्लास्टिक जैविक प्रतिक्रियाशीलता परीक्षण के लिए यूएसपी <88> का अनुपालन किया गया है।
साफ़-सफ़ाई
यह उत्पाद 21 सीएफआर 210.3(बी)(6) में निर्दिष्ट "गैर-फाइबर रिलीजिंग फिल्टर" की परिभाषा के अनुरूप है।
अप्रत्यक्ष खाद्य योज्य
सभी घटक सामग्रियां अप्रत्यक्ष खाद्य योजकों के लिए यूएस एफडीए 21 सीएफआर 177-182 आवश्यकताओं का अनुपालन करती हैं।
भोजन के संपर्क में आने वाली सामग्रियों के लिए सभी घटक सामग्रियां ईयू विनियमन 1935/2004/ईसी का अनुपालन करती हैं।
अधिकतम विभेदक दबाव
20°C पर 3.0 बार के आगे के अंतर दबाव को सहन करता है।
बंध्याकरण विधि
नम गर्मी नसबंदी के 60 चक्रों (121 डिग्री सेल्सियस, 30 मिनट) के बाद, फ़िल्टर अखंडता परीक्षण योग्य रहता है।
बैच रिलीज़ मानदंड
विनिर्माण बैच से नमूने लिए गए फ़िल्टर निम्नलिखित परीक्षणों से गुजरते हैं:
सत्यनिष्ठा परीक्षण
प्रत्येक फ़िल्टर ने जीवाणु चुनौती परीक्षण से संबंधित एक गैर-विनाशकारी अखंडता परीक्षण पास कर लिया है।
बबल प्वाइंट ≥ 60% आईपीए में 1.1 बार: 40% शुद्ध पानी।

 आपका संदेश 20-3,000 अक्षरों के बीच होना चाहिए!
आपका संदेश 20-3,000 अक्षरों के बीच होना चाहिए! कृपया अपनी ईमेल देखें!
कृपया अपनी ईमेल देखें!  आपका संदेश 20-3,000 अक्षरों के बीच होना चाहिए!
आपका संदेश 20-3,000 अक्षरों के बीच होना चाहिए! कृपया अपनी ईमेल देखें!
कृपया अपनी ईमेल देखें!